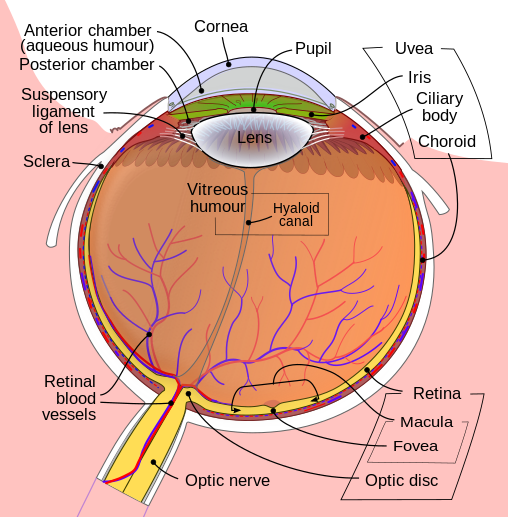
|
It is commonly believed that Dr. Dan-Eric Nilsson and Dr. Susanne Pelger of Lund University in Sweden demonstrated in a scientific paper written back in 1994 that a fully-developed vertebrate eye could have developed from a simple light-sensitive spot by a process of unguided natural selection, in “less than 364,000 years.” That, at any rate, is the popular myth. What’s the reality?
Nilsson and Pelger certainly made a convincing case for gradualism in their paper, but they failed to bolster the case for Darwinism. Looking at the eye from a purely anatomical standpoint, they showed how a vertebrate eye could have developed from a patch of light-sensitive skin by the accumulation of numerous tiny modifications over the course of time – in other words, gradual change, or evolution. But what very few people realize is that Nilsson and Pelger used intelligently guided evolution to transform their flat, light-sensitive spot into a focused camera-type eye. What’s more, Nilsson – who is a convinced Darwinist – has recently acknowledged as much! (I’ll have a lot more to say on that surprising story, below.) Additionally, Nilsson and Pelger’s claim that the eye could have evolved in “less than 364,000 years” turns out to be a hypothetical estimate, which (as we’ll see) only applies to an intelligently designed fitness landscape.
In this post, I’m going to critically examine Nilsson and Pelger’s 1994 paper, A Pessimistic Estimate of the Time Required for an Eye to Evolve (Proceedings: Biological Sciences, Vol. 256, No. 1345, (April 22, 1994), pp. 53-58). Before I continue, I would like to personally thank Professor Dan-Eric Nilsson for having responded to my queries regarding his paper. Dr. Nilsson was also kind enough to send me a copy of his new paper, entitled, “Eye evolution and its functional basis” (forthcoming in Visual Neuroscience, 2013, 30, doi:10.1017/S0952523813000035), which addresses the evolution of the eye in much greater depth than the 1994 paper which he co-authored with Dr. Susanne Pelger. I’m going to discuss Nilsson’s new paper in my next post. I’d also like to thank Dr. Anders Garm, a colleague of Dr. Nilsson, for having taken the time to answer my queries about vision in box jellyfish, in relation to the evolution of the eye.
Does Nilsson and Pelger’s model lend support to Intelligent Design or Darwinian evolution, or both?
It is my contention that Nilsson and Pelger’s model of the evolution of the eye is in fact a striking example of Intelligent Design, rather than Darwinian evolution. Readers may be surprised to learn that Nilsson and Pelger deliberately selected each of the 1,829 steps in their model leading from a light-sensitive spot to a camera-type vertebrate eye, by choosing which features they wanted to vary at every step along the way. That makes their model intelligently designed. And although the pathway created by Nilsson and Pelger was indeed a gradual one, their model lacks a key ingredient that would, if present, turn it into a powerful argument for Darwinism: probability calculations, showing how likely it was that Nature would have chosen the pathway they selected. I conclude that while Nilsson and Pelger’s model can be viewed as an Intelligent Design hypothesis regarding how the eye might have emerged over time through a process of guided evolution, it cannot be legitimately invoked as an argument for accepting Darwinism.
Some readers might object that Nilsson and Pelger’s 364,000-year estimate of the time required for the vertebrate eye to have developed does make it a truly Darwinian model, since it shows that the eye could have evolved relatively quickly. However, it turns out that Nilsson and Pelger’s estimate is based on a host of simplifying assumptions which render it useless for practical purposes. Nilsson and Pelger have left us with a theoretical model of how the eye could have evolved gradually, but without a realistic estimate that would demonstrate the feasibility of their model, over geological time.
Darwinism and the epistemic bar

|
Gold medal winner Ethel Catherwood of Canada scissors over the bar at the 1928 Summer Olympics. Image courtesy of Wikipedia.
For some time now, Darwinists have been fighting – and generally winning – arguments against critics who contended that Darwinian evolution was impossible. They have won these arguments in two ways: firstly, by identifying a scientific flaw in their critics’ assumptions, which either invalidates their anti-Darwinian arguments or calls them into question; and secondly, by constructing theoretical models showing that a step-by-step evolutionary sequence from a hypothetical ancestor to its modern-day descendants would have been viable at each and every stage, and that each step along the way would have conferred a fitness advantage on the creature possessing it. (Nilsson and Pelger’s 1994 paper falls into the second category.) Both of these tactics have served well to establish the theoretical possibility of Darwinism, as a scientific theory.
These tactics by Darwinists certainly make for splendid PR coups, but what do they actually prove? At the very most, all they prove is that Darwinism is theoretically possible: it might (in a very weak sense of “might”) have happened. But theoretical possibility and scientific plausibility are two very different things. In order for a hypothesis to attain the status of a respectable scientific theory, the mechanisms to which it appeals have to clear a certain threshold of probability, before that hypothesis can be deemed scientifically plausible.
What I’m arguing here is that Darwinism has secured public (and scientific) acceptance by lowering the epistemic bar from the standard usually required of a scientific theory. Most theories gain acceptance only after it has been shown that they are scientifically plausible, in addition to being supported by powerful evidence in their favor. For Darwinism, however, this requirement was waived. After making a strong scientific case that his theory of evolution was supported by converging lines of circumstantial evidence, Darwin managed to win acceptance for his new theory, simply by mounting an argument showing that its mechanism (natural selection) was theoretically possible. This was due in no small part to Darwin’s rhetorical skills: in terms of sheer eloquence, his Origin of Species was unmatched in the annals of scientific literature.
Now, there are a couple of significant exceptions to the requirement that a scientific hypothesis should invoke a plausible mechanism before it can be taken seriously as a scientific theory. Newton’s theory of gravity gained acceptance despite the fact that it lacked a known mechanism, for the simple reason that it could be experimentally verified, at scales ranging from falling apples to the movement of the planets in the solar system. And in our own day, no geologist doubts the reality of continental drift, even though the underlying mechanism – plate tectonics – remains poorly understood. After all, scientists can actually measure the continents drifting, at the rate of several centimeters a year. It is easy to extrapolate back in time and show that at one point, they would have all been together. Unfortunately, neither of these exceptions is of any help to Darwinism.
First, the parallel with Newton’s theory of gravity fails. While there is evidence in the fossil record for large-scale evolutionary change, this evidence tells us nothing about the mechanism involved. Hence it cannot be adduced as evidence for Darwinism.
That leaves us with extrapolation. Darwinian evolutionists have long argued that their theory can be validated by extrapolating from observed evolutionary changes, such as those wrought by artificial selection. As Professor Jerry Coyne put it in Nature magazine in 2001:
When, after a Christmas visit, we watch grandma leave on the train to Miami, we assume that the rest of her journey will be an extrapolation of that first quarter-mile. A creationist unwilling to extrapolate from micro- to macroevolution is as irrational as an observer who assumes that, after grandma’s train disappears around the bend, it is seized by divine forces and instantly transported to Florida. (Nature 412:587, 19 August 2001.)

|
A Southbound Amtrak 139 train on the Siver Star route crosses Central Boulevard in downtown Orlando, Florida. Image courtesy of Wikipedia.
What this analogy overlooks is that there is only one railway track taking Grandma to Florida. Macroevolution, on the other hand, is a multi-forked path with an astronomically large number of possible branches, and additionally, there is no intelligent driver and no pre-programmed destination, in Darwin’s theory. Thus in order to demonstrate the feasibility of a vertebrate eye evolving from a light-sensitive spot by a Darwinian mechanism, it is necessary to show first that the probability of its arriving at a vertebrate eye within the time available exceeds a certain threshold, rendering the theory plausible and hence worthy of scientific credence. In other words, proponents of Darwinian evolution have to come up with some “hard numbers.”
Historically, Darwinists have usually resisted this demand for “hard numbers” by arguing that the mathematical calculations verifying the ability of natural selection to transform a light-sensitive spot into a camera-type eye over millions of years were just too hard to do, and that the skeptics’ demand for “hard numbers” was an epistemically unreasonable one. “We’ve got the fossils, as well as the evidence from homology, embryology, vestigial organs and biogeography, and we’ve got a mechanism which has been shown to work on short time-scales,” say the Darwinists. “That should be enough to convince you.”
Nilsson and Pelger’s 1994 paper is therefore highly significant, because it represents a genuine scientific attempt to engage the Darwin-skeptics on their own turf. The authors examine the vertebrate eye – a complex organ often cited by Darwin-skeptics as beyond the power of natural selection to explain – and argue that a step-wise sequence of anatomical changes, occurring one at a time, could have transformed a light-sensitive spot into a fully fledged camera-type eye over a period that they calculate – using conservative assumptions – as “less than 364,000 years,” which means that there was enough time for eyes to evolve 1,500 times over, during the 540 million years since eyes first appeared, back in the Cambrian period. Darwinism wins by a knock-out, right?
Not quite. Unfortunately for Darwinists, the evolution that Nilsson and Pelger describe in their paper is intelligently guided evolution. And I have the evidence to prove it.
Why the model described in Nilsson and Pelger’s 1994 paper is really an example of intelligently guided evolution
The evidence for my contention that Nilsson and Pelger’s model is really an example of intelligently guided evolution comes from two sources: a recent communication sent to me by Professor Nilsson, and the original 1994 paper authored by Nilsson and Pelger.
(a) What Professor Nilsson said recently about the model he and Dr. Pelger created
I claimed above that Professor Nilsson had recently acknowledged that the step-by-step sequence from a light-sensitive spot to a camera eye, which he and Dr. Pelger described in described in their 1994 paper, was actually an intelligently guided evolutionary sequence. I’m now going to supply “chapter and verse” to back up that claim. I recently contacted Professor Nilsson, and asked him about the 1994 paper he co-authored with Susanne Pelger, and he was kind enough to respond. In his response, he patiently explained to me exactly what he and Pelger were trying to establish with their model:
If there is random and heritable variation separately controlling a large number of different parameters, then selection would, for each generation, choose the route that causes the largest improvement of whatever selection was set to favour (e.g. acuity). So, if there is more than one parameter that can vary, evolution can be expected to follow different routes depending on how much the different parameters vary, and how much impact they have on acuity. In real eye evolution, there would be numerous different parameters that express heritable variation in the population, and the amount of variation could itself be modified by selection. Real eye evolution has also resulted in many different end products, using different ways to form images, and different cellular organizations to obtain particular structures. These are all interesting questions, but the Nilsson and Pelger 94 paper does not address these questions. Instead the paper asks the much more tenable question: is there a continuous route from a flat, non-imaging light detector to a focused camera type eye, where each little modification, no matter how small, would generate an improvement in acuity. The important answer we find is yes, there is at least one such route. Although this route was devised by us (by deciding which parameters were to change during different phases along the route) the important result is that there is at least one route where acuity continuously improves at each new generation. Real evolution may find an even shorter route, where acuity improves more for each generation, but that would not change the important conclusion that an eye can evolve from a patch of light sensitive skin by numerous tiny modifications.
Referring to his model of how the eye evolved, Nilsson admits that “this route was devised by us,” in order to generate “a continuous route from a flat, non-imaging light detector to a focused camera type eye, where each little modification, no matter how small, would generate an improvement in acuity,” and that it was constructed “by deciding which parameters were to change during different phases along the route.” That’s intelligently guided evolution. No two ways about it. Case closed.
(b) What Nilsson and Pelger said about their model in their 1994 paper
The fact that Nilsson and Pelger relied on intelligently guided evolution to generate a vertebrate eye from a flat light-sensitive spot shouldn’t be news to anyone who has taken the trouble to read their 1994 paper, A pessimistic estimate of the time required for an eye to evolve (Proceedings: Biological Sciences, Vol. 256, No. 1345, April 22, 1994, pp. 53-58). The clues were there all along. Here’s what Nilsson and Pelger wrote about their mathematical model, in their paper:
Estimates of the number of generations required to make a change to a simple quantitative character are easily made if the phenotypic variation, selection intensity and heritability of the character are known (Falconer, 1989). The evolution of complex structures, however, involves modifications of a large number of separate quantitative characters, and in addition there may be discrete innovations and an unknown number of hidden but necessary phenotypical changes. These complications seem effectively to prevent evolution rate estimates for entire organs and other complex structures. An eye is unique in this respect because the structures required for image formation, although there may be several, are all typically quantitative in their nature, and can be treated as local modifications of pre-existing tissues. taking a patch of light-sensitive epithelium as the starting point, we avoid the more inaccessible problem of photoreceptor cell evolution (Goldsmith 1990; Land and Fernald 1992). Thus if the objective is limited to finding the number of generations required for the evolution of the eye’s optical geometry, then the problem becomes solvable.
We have made such calculations by outlining a plausible sequence of alterations leading from a light-sensitive spot all the way to a fully-developed lens eye. The model sequence is made such that every part of it, no matter how small, results in an increase in the spatial information the eye can detect. The amount of morphological change required for the whole sequence is then used to calculate the number of generations required. Whenever plausible values had to be assumed, such as for selection intensity and phenotypic variation, we deliberately picked values that overestimate the number of generations. Despite this consistently pessimistic approach, we arrive at only a few hundred thousand generations!…
The first and most crucial task is to work out an evolutionary sequence which would be continuously driven by selection. The sequence should be consistent with evidence from comparative anatomy, but preferably without being specific to any particular group of animals. Ideally we would like selection to work on a single function throughout the sequence. Fortunately, spatial resolution, i.e. visual acuity, is just such a fundamental aspect and it provides the role reason for the eye’s optical design (Snyder et al., 1977; Nilsson 1990; Warrant and Macintyre 1993)…
The refractive index of the vitreous lens is assumed to be 1.35.… The aperture size in Stage 6 was chosen to reflect the typical proportions in real eyes of this type.
A graded-index lens can be introduced gradually as a local increase of refractive index…
Based on the principles outlined above, we made a model sequence of which representative stages are shown in figure 2. [The enclosed figure shows eight distinct stages, out of a total of 1,829 in their model – VJT.] The starting point is a flat light-sensitive epithelium, which by invagination forms the retina of a pigmented pit eye. After the constriction of the aperture and the gradual formation of a lens, the final product becomes a focused camera-type eye with the geometry typical for aquatic animals (e.g. fish and cephalopods).
To sum up: Nilsson and Pelger started out with a flat, light-sensitive spot, whose dimensions and thickness they were able to describe with the aid of a few mathematical parameters. They then planned a continuous route from a flat, non-imaging light detector to a focused camera-type eye, such that each little modification, no matter how small, would generate an improvement in visual acuity. The evolutionary sequence was not generated by some random process; it was planned in every detail, at every step by Nilsson and Pelger. They decided “which parameters were to change during different phases along the route.”

|
Schematic diagram showing the evolution of the eye. Image courtesy of Matticus78 and Wikipedia.
Nilsson and Pelger’s model certainly is a gradualistic model, but it cannot be called a Darwinian model, because although the model’s authors created “an evolutionary sequence which would be continuously driven by selection,” they made no attempt to quantify the likelihood of those changes occurring, in that particular sequence, without intelligent guidance. Without that probability calculation, Nilsson and Pelger cannot claim to have shown that their hypothetical model supports Darwinian evolution. As Nilsson himself put it in his recent communication to me, “this route was devised by us.”
While Nilsson and Pelger can rightly claim to have demonstrated in their 1994 paper is the theoretical possibility of the eye evolving in a Darwinian fashion, at the morphological level, the claim the authors make in the final sentence of their paper, that “the eye was never a real threat to Darwin’s theory of evolution,” remains a doubtful one. Theoretical possibility is not enough to render a theory scientifically plausible.
How Nilsson and Pelger’s 1994 paper has been mis-reported by evolutionists over the last two decades

|

|
Left: Professor Richard Dawkins at the 34th American Atheists Conference in Minneapolis, on 21 March 2008. Image courtesy of Mike Cornwell and Wikipedia.
Right: Professor Jerry Coyne. Image courtesy of Wikipedia.
Ever since Nilsson and Pelger’s paper was published, Darwinian evolutionists citing the paper have almost invariably mis-reported its findings. Two great myths have been recycled in the literature again and again: the fiction that Nilsson and Pelger’s model was a computer simulation, and the fiction that the variations in the model were random, like the variations in Darwinian evolution. We now know – thanks to the indefatigable research of Dr. David Berlinski – is that Nilsson and Pelger’s model didn’t even use a computer. And now we also know that the variations introduced into the model were deliberately designed, rather than random.
(a) The myth of the computer simulation – how it all got started, and how it continues to be perpetuated
The reader may be wondering: how did these myths get propagated in the first place? The answer is that they originated from an eminent scientist whom no-one dared to publicly contradict when he got it wrong. I’m referring, of course, Professor Richard Dawkins, who completely mis-read what Nilsson and Pelger said in their 1994 paper. (I wish to state here that I am not accusing Professor Dawkins of acting in bad faith; it is entirely possible that he misconstrued Nilsson & Pelger’s study. In that case, Dawkins is guilty of incompetence, rather than deceit.) In a jubilant review of Nilsson and Pelger’s paper, “The Eye in a Twinkling” (Nature Vol. 368, 21 April 1994, pp. 690-691), Dawkins claimed that their model was based on not one but two computer simulations, and that the mutations occurring in their model were random:
“Nilsson and Pelger’s task was to set up two computer models of evolving eyes to answer two questions. First, is there a smooth trajectory of change, from flat skin to full camera eye, such that every intermediate is an improvement? (Unlike human designers, natural selection can’t go downhill, not even if there is a tempting higher hill the other side of the valley.) Second, how long would the necessary quantity of evolutionary change take?
“Nilsson and Pelger worked at the level of tissue deformations, not at the level of cellular biophysics. The existence of a light-sensitive cell was taken as a given…
“Nilsson and Pelger began with a flat retina, atop a flat pigment layer and surmounted by a flat, transparent layer (see figure). The transparent layer was allowed localized random mutations of its refractive index. They then let the model deform itself at random, constrained only by the requirement that any change must be only 1% bigger or smaller than what went before. And, of course, in order for a change to be accepted, it had to be an improvement on what went before.
“The results were swift and decisive. A trajectory of steadily improving acuity led unhesitatingly from a flat beginning through a shallow cup to a steadily deepening cup. The transparent layer thickened to fill the cup and smoothly curved its outer surface. And then, almost like a conjuring trick, a portion of this transparent filling condensed into a local, spherical subregion of higher refractive index – not uniformly higher, but a gradient of refractive index such that the spherical region functioned as an excellent graded-index lens. Best of all, the ratio of the focal length of the lens to its diameter settled down at a close approximation to Mattiessen’s ratio, long known to be the ideal value for a graded-index lens.
“Turning to the question of how long the evolution might have taken, Nilsson and Pelger had to make some plausible population-genetic assumptions. They chose values of heritability, coefficient of variation and intensity of selection from published observations in the field. Their guiding principle in choosing such numbers was pessimism. For each assumption they made, they wanted to err in the direction of overestimating the time taken for the eye to evolve. They even went so far as to assume that any new generation differed in only one part of the eye: simultaneous changes in different parts of the eye, which would have speeded up evolution, were banned. But even with these conservative assumptions, the time taken for a fish eye to evolve from flat skin was under 400,000 generations. Assuming typical generation times of one year for small animals, the time needed for the evolution of the eye, far from stretching credulity with its vastness, turns out to be too short for geologists to measure. It is a geological blink.”
Professor Richard Dawkins’ egregious misreading of Nilsson and Pelger’s paper has been roundly criticized by mathematician David Berlinski. Berlinski is the author of several articles exposing the scientific mis-reporting of Nilsson and Pelger’s paper, including A Scientific Scandal (Commentary, March 31, 2001), The Vexing Eye (Commentary, February 12, 2003), and A Scientific Scandal? David Berlinski & Critics (Commentary, July 8, 2003):
“Nilsson and Pelger’s paper has gained currency in both the popular and the scientific press because it has been misrepresented as a computer simulation, most notably by Richard Dawkins. Word spread from Dawkins’s mouth to any number of eagerly cupped but woefully gullible ears. Subsequent references to Nilsson and Pelger’s work have ignored what they actually wrote in favor of that missing computer simulation, in a nice example of a virtual form of virtual reality finally displacing the real thing altogether…
In a more recent paper entitled, The Vampire’s Heart, Berlinski took up the issue again, in an online response to James Downard:
The facts: Nilsson & Pelger’s study, which was widely considered a computer simulation, contained no computer simulation whatsoever. It contained, in fact, no computer analysis at all, perhaps because it contained no analysis at all. It was Richard Dawkins who conveyed the widespread impression to the contrary, writing about a computer simulation that did not exist with the excitement of a man persuaded that he had seen a digital vision. As, indeed, he had. Commentators at the time came to Dawkins’ defense with a gratifyingly prompt display of personal generosity, so that what was, in fact, a complete fabrication took on the aspects of an understandable but trivial error. Any man, after all, might mistake nothing for something.
The “computer simulation” myth continues to be propagated, right down to the present day. In a recent blog article on Nilsson and Pelger’s paper, entitled, Evolution of the Eye: Nilsson & Pelger and Lens Evolution (January 22, 2011), the neo-Darwinist blogger Francis Smallwood (who is also a good friend of ID proponent Joshua Gidney) referred to an “evolutionary simulation” created by Nilsson and Pelger, which was “not programmed to progress in ever-improving stages,” but which “allowed mutation” from which the fittest variation was selected – in other words, “true natural selection”:
Nilsson and Pelger postulated there being three types of tissue of which the eye was comprised: an opaque shield which covered the back of the eye; photocells; and a transparent film or substance (an example of this would be the vitreous mass which we looked at in the previous post.) An eye endowed with the previous constitution formed the basis from which their evolutionary simulation would begin…
So, how did N&P [Nilsson and Pelger] intend their computer eyes to evolve? They treated a genetic mutation as a percentage change in a certain part of the eye, for example, a decrease in the thickness of the transparent layer. A mutation would affect the size of part of the eye, or the functional quality of a part of the eye, such as the refractive index (which we will come to later). And, importantly, the simulation was not programmed to progress in ever-improving stages, as if the whole evolutionary progression was pre-programmed and they simply divided the one long evolutionary phase into lots of small phases, chopping up a pre-selected evolutionary progression into small quantifiable, arbitrary units. Instead, they allowed mutation from which would be selected the variations (mutations) which improved the computer eye – true natural selection…
By allowing mutation in the refractive index of the computer eye, and thus allowing variation which selection could then act upon. The single criterion which selection solicited was improved eyesight. If this criterion was met, however fractionally, selection would harness it and further “act” upon it.
Unfortunately, Smallwood has mis-read Nilsson and Pelger’s paper. Nilsson and Pelger clearly indicate in their paper that the mutations occurred in a planned order (folding mutations first, followed by aperture construction mutations, followed by mutations that varied the refractive index of the lens, followed by mutations that varied the shape and size of the lens), and in his recent communication to me, Professor Nilsson added that “this route was devised by us (by deciding which parameters were to change during different phases along the route).” Whichever way you slice and dice it, that’s not natural selection. That’s intelligently guided evolution.
(b) The myth of the randomly varying parameters

|
The second great myth which I drew attention to above was the mistaken notion that the variations introduced into Nilsson and Pelger’s model were random ones, when in fact they were all painstakingly designed. This myth also goes back to Professor Richard Dawkins’ review of Nilsson and Pelger’s paper, “The Eye in a Twinkling” (Nature Vol. 368, 21 April 1994, pp. 690-691), in which he wrote:
Nilsson and Pelger began with a flat retina, atop a flat pigment layer and surmounted by a flat, transparent layer (see figure). The transparent layer was allowed localized random mutations of its refractive index. They then let the model deform itself at random, constrained only by the requirement that any change must be only 1% bigger or smaller than what went before. And, of course, in order for a change to be accepted, it had to be an improvement on what went before.
Dawkins went on to breathlessly inform his readers that the results of these random variations were magical: “almost like a conjuring trick, a portion of this transparent filling condensed into a local, spherical subregion of higher refractive index – not uniformly higher, but a gradient of refractive index such that the spherical region functioned as an excellent graded-index lens.”
The myth that Nilsson and Pelger’s model employed random variations was deftly taken apart by the mathematician Dr. David Berlinski in his paper, A Scientific Scandal? David Berlinski & Critics (Commentary, July 8, 2003), from which I shall quote a brief excerpt:
“…[T]he flaw in Nilsson and Pelger’s work to which I attach the greatest importance is that, as a defense of Darwinian theory, it makes no mention of Darwinian principles. Those principles demand that biological change be driven first by random variation and then by natural selection. There are no random variations in Nilsson and Pelger’s theory. Whatever else their light-sensitive cells may be doing, they are not throwing down dice or flipping coins to figure out where they are going next…
Regrettably, Professor Jerry Coyne (who should know better) continues to propagate this myth in his best-selling 2009 book, Why Evolution Is True:
“We can, starting with a simple precursor, actually model the evolution of the eye and see whether selection can turn that precursor into a more complex eye within a reasonable amount of time. Dan Nilsson and Susanne Pelger of Lund University in Sweden made such a mathematical model, starting with a patch of light-sensitive cells backed by a pigment layer (a retina). They then allowed the tissues around this structure to deform themselves randomly, limiting the amount of change to only 1% of size or thickness at each step. To mimic natural selection, the model accepted only mutations that improved the visual acuity, and rejected those that degraded it.
“Within an amazingly short time, the model yielded a complex eye, going through stages similar to the real-animal series described above. The eyes folded inward to form a cup, the cup became capped with a transparent surface, and the interior of the cup gelled to form not only a lens, but a lens with dimensions that produced the best possible image.
“Beginning with a flatworm-like eyespot, then, the model produced something like the complex eye of vertebrates, all through a series of tiny adaptive steps – 1,829 of them, to be exact. But Nilsson and Pelger could also calculate how long this process would take. To do this, they made some assumptions about how much genetic variation for eye shape existed in the population that began experiencing selection, and how strongly selection would favor each useful step in eye size. These assumptions were deliberately conservative, assuming that there were reasonable but not large amounts of genetic variation and that natural selection was very weak. Nevertheless, the eye evolved very quickly: the entire process from rudimentary light-patch to camera eye took fewer than 400,000 years.
– Coyne, Jerry A. Why Evolution Is True, 2009, Oxford University Press, p. 155.
In the above passage, Coyne carefully avoids repeating Dawkins’ ridiculous assertion that Nilsson and Pelger’s model was created by a computer simulation, referring only to a “mathematical model.” However, he perpetuates a popular misunderstanding of that model when he claims that Nilsson and Pelger “allowed the tissues … to deform themselves randomly,” and that these random variations, coupled with the culling action of natural selection in rejecting mutations that diminished visual acuity, yielded “a complex eye” within “an amazingly short time.”
What Coyne overlooks here is that the mutations in Nilsson and Pelger’s model were anything but random: each and every one of them was designed by the model’s authors. The authors did indeed manage to trace a viable morphological path from a flat light-sensitive spot to a vertebrate eye, but they failed to show that unguided natural selection had a reasonable probability (within the time available) of finding and successfully traversing this lucky path, without getting side-tracked down an evolutionary blind-alley. For this reason, Nilsson and Pelger’s model, by itself, proves nothing about the ability of natural selection to bring about macroevolutionary transformations.
How did Nilsson and Pelger arrive at their 364,000-year time estimate for the evolution of the eye?
For those who are mathematically inclined, Nilsson and Pelger’s 364,000-year estimate of the time required for the eye to evolve was derived as follows. After identifying the mathematical principles governing the camera eye (which is found in vertebrates and many cephalopods), Nilsson and Pelger constructed a model sequence, starting with “a flat patch of light-sensitive cells sandwiched between a transparent protective layer and a layer of dark pigment” and finishing with a camera eye possessing a spherical graded-index lens, a flat iris and a focal length that allowed it to focus images sharply. Eight of the steps in Nilsson and Pelger’s model sequence are illustrated in Figure 2 of their paper. However, there were in fact 1,829 steps altogether, owing to the constraint imposed by the authors, that the amount of change in size or thickness at each step would be limited to only 1%. Now, Nilsson and Pelger’s model of the eye described it in terms of about ten features which could be measured mathematically: corneal width, corneal thickness, upper retinal surface width, lower retinal surface width, upper pigment surface width, lower pigment surface width, central refractive index, iris width, lens width and lens height. The fact that there are no less than ten mathematical parameters might appear to make modeling very difficult, but in fact, if there is more than one parameter that changes, it is possible to treat the changes in the different parameters as if they were all changes in a single parameter, and then sum the number of 1% steps for the different kinds of changes taking place, in order to arrive at a total measure of change. Now if we take a 1% change and cumulate it 1,829 times we get something roughly equivalent to an 80 million-fold change, as (1.01)^1829 equals 80,129,540. In other words, the total amount of change in the ten different mathematical parameters describing the eye is equivalent to an 80 million-fold change in just one of these parameters. For those readers who are accustomed to thinking in visual terms, Nilsson and Pelger provided a helpful analogy:
“In terms of morphological modification, the evolution of an eye can thus be compared to the lengthening of a structure, say a finger, from a modest 10 cm to 8,000 km, or a fifth of the Earth’s circumference” (p. 56).
Quite some change, one might think! So how long would it take to accomplish? At this point in their paper, Nilsson and Pelger referred to an equation which is commonly used to calculate the observable change in each generation:
R = (h^2).i.V.m
where R is the response, or the observable change in each generation, (h^2) is the heritability (i.e. the proportion of phenotypic variance which is genetically determined), i is the intensity of selection, V is the coefficient of variation (i.e. the ratio between the standard deviation and the mean in a population), and m is the mean in a population. Nilsson and Pelger assigned a value of 0.50 to (h^2), which they say is a standard value. They then (pessimistically) assumed that i = 0.01 and V = 0.01, making the right hand side of their equation equal to 0.5 times 0.01 times 0.01 times the mean, m, or in other words 0.00005 times the mean, m.
It follows that even though the genetic changes occurring in mutated individuals are 1% changes, the observed changes occurring in the population as a whole is only 0.005% per generation (or 0.00005 times the mean, m). Nilsson and Pelger then reasoned as follows:
The number of generations, n, for the whole sequence is then given by 1.00005^n = 80,129,540, which implies that n = 363,992 generations would be sufficient for a lens eye to evolve by natural selection.… (p. 57)
If we assume a generation time of one year, which is common for small and medium-sized aquatic animals, it would take less than 364,000 years for a camera eye to evolve from a light-sensitive patch. (p. 58)
Eight reasons for taking Nilsson and Pelger’s time estimate for the evolution of the eye with a grain of salt
There are several good reasons for refusing to take Nilsson and Pelger’s 364,000-year estimate seriously, as a calculation for how long it would take for the vertebrate eye to evolve. In the absence of a credible estimate, Nilsson and Pelger’s model can no longer be said to buttress the case for Darwinism. All it establishes is that the eye could have developed from a much simpler structure, via a gradual process – and the only gradual process which the authors have shown to be capable of generating an eye is an intelligently guided one.
Here, then, are my eight reasons for skepticism, regarding the 364,000-year estimate.
(a) The 364,000-year figure is a “nice round number,” which appears to have been deliberately chosen in order to provide Darwin’s theory with some good publicity

|
Mask of Tutankhamun’s mummy at the Egyptian Museum in Cairo. Image courtesy of Bjorn Christian Torrissen and Wikipedia.
My first reason for skepticism is that the 364,000-year figure cited by Nilsson and Pelger in their paper is what I would call a “nice round number.” It sounds even better when you express it in approximate terms, as “a few hundred thousand years,” as Nilsson and Pelger do in their summary. It’s a number which gives every indication of having been carefully crafted in order to impress laypeople with the creative power of natural selection, without exciting their incredulity. Most people think of evolution as a long process that takes millions of years. When we are told that the eye evolved in only 300,000 years, it sounds fast, because it’s well under the “magic million” mark. At the same time, it doesn’t sound too fast, as a figure of, say, 3,000 years would be. Nobody would believe a figure like that.
However, what the evolutionists who cite Nilsson and Pelger’s 364,000-year estimate overlook is that it was deliberately intended to be a conservative figure. That’s why the title reads: “A Pessimistic Estimate of the Time Required for an Eye to Evolve.” As we saw above, Nilsson and Pelger assigned a value of 0.01 to the intensity of selection i, and they also selected a value for 0.01 for the coefficient of variation V. However, each of these figures could have been plausibly chosen to be 10 times higher. Setting the intensity of selection i to 0.01 means that a randomly chosen organism in the general population would have a chance of survival that’s 99% as high as that of an organism possessing the favorable mutation – a very weak selection effect indeed. By comparison, this table, which is taken from the online notes for a university course in population genetics, lists values for the selection intensity i ranging from 0.00 to 2.67. Thus Nilsson and Pelger could have easily assigned a value of 0.10 to i, instead of 0.01.
Likewise, Nilsson and Pelger’s estimate of 0.01 for the coefficient of variation V is very modest: according to some online course notes on Fitness, Adaptation, and Natural Selection in Real Populations by Dr. Steven Carr (Department of Biology, Memorial University of Newfoundland, Canada) a value of 5% to 10% (0.05 to 0.10) for V is typical “for many traits in many organisms.” Had Nilsson and Pelger used a value of 0.10 for the intensity of selection i and the coefficient of variation V, their calculation would have shown that the eye could have evolved in just 3,650 years, which is roughly equivalent to the time that has elapsed since the death of Pharaoh Tutankhamun, who ruled Egypt from 1332 to 1323 B.C.
Here’s my challenge to Darwinian biologists: try telling the man-in-the-street that the vertebrate eye could have evolved from a light-sensitive spot in the time since King Tut died, and see what kind of reaction you get. I wouldn’t mind betting that it will weaken, rather than strengthen, his belief in Darwin’s theory of evolution.

|

|
Left: An okapi at Marwell Wildlife, Hampshire, England. Image courtesy of Cahrles Miller and Wikipedia.
Right: A giraffe at Mikumi National Park, Tanzania. Image courtesy of Muhammad Mahdi Karim and Wikipedia.
But there’s an even simpler way to illustrate the biological absurdity of Nilsson and Pelger’s 364,000-year estimate of the time required for the eye to evolve. Let’s go back to what Nilsson and Pelger said in their paper about the changes that occurred in the evolution of the vertebrate eye, from a light-sensitive spot:
In terms of morphological modification, the evolution of an eye can thus be compared to the lengthening of a structure, say a finger, from a modest 10 cm to 8,000 km, or a fifth of the Earth’s circumference” (p. 56).
Let’s now consider a much smaller change: the lengthening of the giraffe’s neck. Above is a picture of a giraffe, next to its closest living relative, the okapi. The structure we are considering here is the giraffe’s neck, which is less than 10 times as long as an okapi’s. Ask the man-in-the-street how long it took for the giraffe to get its long neck, and he’ll probably say, “A few million years.” And yet, we are supposed to believe that the changes involved in transforming a light-sensitive spot into a vertebrate eye, which are equivalent to an 80 million-fold lengthening of an animal’s neck, could have taken place in 364,000 years?
(b) Nilsson and Pelger admit that their estimate is a purely hypothetical one
Second, Nilsson and Pelger explicitly acknowledge in the final paragraph of their paper that their 364,000-year estimate was never meant to be a realistic one, and applies to a hypothetical situation in which “selection for eye geometry and optical structures imposed the only limit.” As they put it:
Because eyes cannot evolve on their own, our calculations do not say how long it actually took for eyes to evolve in the various animal groups. However, the estimate demonstrates that eye evolution would be extremely fast if selection for eye geometry and optical structures imposed the only limit. This implies that eyes can be expected to respond very rapidly to evolutionary changes in the lifestyle of a species. (p. 58)
However, the only situation in which selection for eye geometry and optical structures are likely to impose the only limit on the rate of evolution is one in which all of the other complex organs of the body have already evolved – which of course begs the question. And that brings me to my next point.
(c) Nilsson and Pelger’s estimate isn’t anatomically realistic: it leaves out the brain
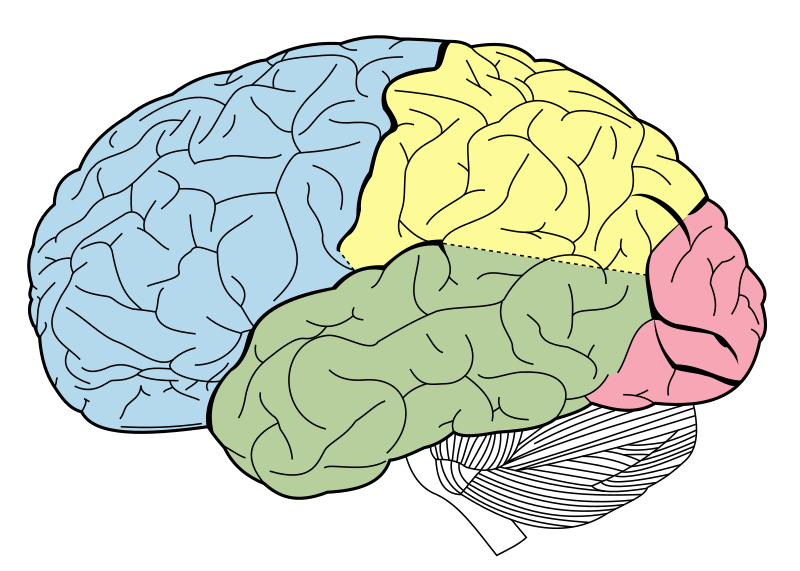
|
The occipital lobe of the human brain (pink). The occipital lobe is the visual processing center of the mammalian brain, containing most of the anatomical region of the visual cortex. Figure 728 from Gray’s Anatomy, with labels removed. Image courtesy of Wikipedia.
Third, Nilsson and Pelger acknowledge in their paper that their 364,000-year estimate for the time required for the evolution of the eye was arrived at by focusing on the evolution of the optical structures of the eye only, in isolation from the brain. But as the authors readily acknowledge, an eye “makes little sense” without an advanced brain, and the complex vertebrate eye of a fish requires a fish brain in order to process the information it conveys to the nervous system:
If advanced lens eyes can evolve so fast, why are there still so many examples of intermediate designs among recent animals? The answer is clearly related to a fact that we have deliberately ignored, namely that an eye makes little sense on its own. Although reasonably well-developed eyes are found even in jellyfish (Piatigorsky et al., 1989), one would expect most lens eyes to be useless to their bearers without advanced neural processing.. For a sluggish worm to take full advantage of a pair of fish eyes, it would need a brain with large optic lobes. But that would not be enough, because the information from the optic lobes would need to be integrated in associative centres, fed to motor centres, and then relayed to muscles in an advanced locomotory system. In other words, the worm would need to become a fish. (p. 58)
Insofar as Nilsson and Pelger’s estimate is derived by focusing on one organ of the body to the exclusion of all others, it cannot be regarded as anatomically realistic.
(d) Nilsson and Pelger’s estimate isn’t ecologically realistic
Fourth, Nilsson and Pelger readily admit in their paper that their 364,000-year estimate deliberately confines its attention to one organism, and ignores changes occurring in other species, and in the organism’s environment:
Additionally, the eyes and all other advanced features of an animal like a fish become useful only after the whole ecological environment has evolved to a level where fast visually guided locomotion is beneficial. (p. 58)
In other words, Nilsson and Pelger’s 364,000-year estimate for the evolution of the eye is not ecologically realistic. It considers one organism in isolation from its surroundings.
(e) Nilsson and Pelger’s estimate isn’t computationally realistic

|
A surface with two local maxima. (Only one of them is the global maximum.) If a hill-climber begins in a poor location, it may converge to the lower maximum. Image courtesy of Wikipedia.
Fifth, Nilsson and Pelger’s 364,000-year estimate for the time required for the optical structures of the eye to evolve assumes a very smooth fitness landscape, as Dov Rhodes demonstrated in a 2007 physics thesis which addressed their 1994 paper. Using a genetic algorithm, Rhodes calculates that Nilsson and Pelger have under-estimated the time required by a factor of at least five, and more realistically fifty. Rhodes’ thesis, which is entitled, Approximating the Evolution Time of the Eye: A Genetic Algorithms Approach, makes for fascinating reading. I’d like to quote a few brief excerpts:
“A paper published in 1994 by the Swedish scientists Nilsson and Pelger [6] gained immediate worldwide fame for describing the evolution process for an eye, and approximating the time required for an eye to evolve from a simple patch that sense electromagnetic radiation. Nilsson and Pelger (NP) outlined an evolutionary path, where by minute improvements on each step a camera type eye can evolve in approximately 360,000 years, which is extremely fast on an evolutionary time scale.… (p. 1)
“The main problem with the NP model is that although the evolutionary path that it describes might be a legitimate one, it neglects consideration for divergent paths. It is easy to construct a situation in which the best temporary option for the improvement of an eye does not lead towards the development of the globally optimal solution. This idea motivates our alternative approach, the method of genetic algorithms. In this paper we use the genetic algorithm with a simplified (2-dimensional) version of NP’s setup and show the error in their approach. We argue that if their approach is mistaken in the simplified model, it is even farther from reality in the full evolutionary setting. (p. 2)
“Although the paraboloid landscape guarantees convergence, the GA [genetic algorithm] is still a probabilistic algorithm and thus will not always converge quickly. As in evolution, the most efficient path is not necessarily the one taken. This fact suggests that our already conservative value of lambda = 5.41 would be even larger if compared with a real deterministic algorithm such as the NP (Nilsson-Pelger) model. Even though their computation accounts to some extent for the average probability of evolutionary development over time, it fails to consider the countless different evolutionary paths, and instead chooses just one.
“Rather than 360 thousand generations, a reasonable lower bound should be at least 5*360,000 = 1.8*10^6 generations, and if our previous speculations have merit, an order of magnitude higher would ramp up the estimate to around 18 million generations. Future experiments that would be useful for improving the accuracy of our results might involve varying the mutation parameter, and most importantly letting algorithms run for longer, allowing the lower bound for convergence to be pushed even higher.” (p. 15)
That was in 2007. I recently emailed Dr. Anders Garm, a collegue of Dr. Nilsson’s, with whom he co-authored a 2011 paper entitled, Box Jellyfish Use Terrestrial Visual Cues for Navigation by Anders Garm, Magnus Oskarsson and Dan-Eric Nilsson (Current Biology 21, 798-803, May 10, 2011. DOI 10.1016/j.cub.2011.03.054). Most of my questions related to vision in jellyfish and other animals, but I also referred in passing to the genetic algorithm described in Dov Rhodes’ 2007 thesis and asked Dr. Garm: “Have any more sophisticated algorithms been developed?” Dr. Garm is a very busy man, but he was gracious enough to issue a brief response that was straight to the point: “No, we do not have such plans at the moment.”
(f) Nilsson and Pelger’s estimate isn’t genetically plausible
Sixth, Nilsson and Pelger say nothing in their paper about the genetic changes required to produce an eye. At the morphological level, the changes look plausible enough; but we have no idea whether continuity at the morphological level translates into continuity at the genetic level. It may, and it may not. We simply don’t know.
The reader will recall that each of the “mutations” in Nilsson and Pelger’s model involved changes of up to 1% in one of ten or so features of the eye: corneal width, corneal thickness, upper retinal surface width, lower retinal surface width, upper pigment surface width, lower pigment surface width, central refractive index, iris width, lens width and lens height. What this assumes, of course, is that there is some gene inside the organism’s DNA which will vary each of these properties, without varying anything else. That’s a convenient simplifying assumption, to be sure, but it’s not realistic at a genetic level. It’s more likely that changes to one of these features will impact – perhaps adversely – on the other features, rendering the organism less viable.
(g) Nilsson and Pelger’s estimate isn’t plausible at the embryological level
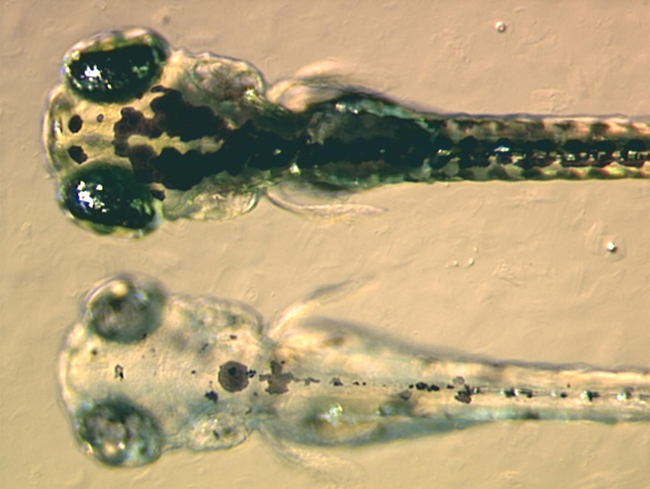
|
Zebrafish embryos. The image at the top is that of a wild-type embryo, while the image at the bottom is that of a zebrafish pigment mutant, which lacks black pigment. Image courtesy of Adam Amsterdam (MIT), J. Bradbury (Small Fish, Big Science) and Wikipedia.
Seventh, Nilsson and Pelger fail to address the question of how the changes required to produce an eye would have impacted the embryonic development of organisms that were evolving this eye. Organisms’ developmental pathways are extremely fragile, especially in the early stages. The idea that changes to these pathways are highly heritable – Nilsson and Pelger suggest a heritability of 50% in their 1994 paper – is biologically implausible. As we’ll see, a more realistic figure for the heritability in such a case would be zero.
The reader will recall that in their paper, Nilsson and Pelger posited the occurrence of no less than 1,829 mutations leading from a creature with a flat light-sensitive spot to a creature with a camera-type vertebrate eye. In estimating the time required for an entire population of organisms to acquire all these mutations, one at a time, they used the following equation to calculate the observable change in each generation:
R = (h^2).i.V.m
where R is the response, or the observable change in each generation, (h^2) is the heritability (i.e. the proportion of phenotypic variance which is genetically determined), i is the intensity of selection, V is the coefficient of variation (i.e. the ratio between the standard deviation and the mean in a population), and m is the mean in a population. Nilsson and Pelger assigned a value of 0.50 to the heritability (h^2), which they said was a common value: in normal cases, they claimed, the heritability is greater than 0.50. They then (pessimistically) assumed that i = 0.01 and V = 0.01, and arrived at a figure of 364,000 years.
I recently emailed a biologist – I won’t reveal his name, as he wishes to preserve his privacy – asking him for his comments on Nilsson and Pelger’s 364,000-year estimate. He replied that for him, speaking as a developmental biologist, the most serious problem with Nilsson and Pelger’s hypothesis was their estimate of the heritability (h^2), for the sort of variation that would be required to transform a flat patch of light-sensitive cells into a hemisphere over many generations.
The biologist whom I contacted argued that while different animals form eyes in various ways, in every case the developmental pathways that culminate in a functional eye are highly constrained. Although there are minor variations in normal eye morphology between individuals in any given species, there is no evidence that the developmental noise represented by those variations is genetically determined. In general, developmental pathways are specified by many more factors than DNA sequences. While DNA is necessary for generating
morphology and physiology, it is far from being sufficient. DNA isn’t everything: there is a lot of other information in the developing embryo that is arguably much more important than the information contained in its DNA. Heritability estimates based on genetic variation simply ignore all these other factors.
The biologist who advised me also pointed out that while heritable genetic mutations are sometimes responsible for some eye defects, none of them came anywhere close to accounting for the elaborate cumulative folding needed for Nilsson and Pelger’s hypothesis to work. In any case, since such defects are harmful, their selection coefficient would actually be negative. He concluded:
So the most realistic value for heritability in this case is 0.00, not 0.50. Zero times anything is zero, so Nilsson & Pelger’s hypothesis is dead from the start.
(h) Nilsson and Pelger’s model isn’t plausible at the biochemical level
Eighth and finally, Nilsson and Pelger fail to address the biochemical changes that must have occurred in the eye, during its evolution from a light-sensitive spot to a vertebrate eye.
Why biochemistry matters, when you are talking about the evolution of the eye

|
Visual phototransduction is a process by which light is converted into electrical signals in the rod cells, cone cells and photosensitive ganglion cells of the retina of the eye. The image above depicts an outer membrane disk in a rod cell. Image courtesy of Jason J. Corneveaux and Wikipedia.
Why, the reader might ask, is biochemistry such fundamental importance, when discussing the evolution of vision? Professor Michael Behe explained why in his 1996 article, Molecular Machines: Experimental Support for the Design Inference:
“The relevant steps in biological processes occur ultimately at the molecular level, so a satisfactory explanation of a biological phenomenon such as sight, or digestion, or immunity, must include a molecular explanation. It is no longer sufficient, now that the black box of vision has been opened, for an ‘evolutionary explanation’ of that power to invoke only the anatomical structures of whole eyes, as Darwin did in the 19th century and as most popularizers of evolution continue to do today. Anatomy is, quite simply, irrelevant.”
To illustrate his point, Professor Behe described the process whereby the human eye sees:
Let us return to the question, how do we see? Although to Darwin the primary event of vision was a black box, through the efforts of many biochemists an answer to the question of sight is at hand. When light strikes the retina a photon is absorbed by an organic molecule called 11-cis-retinal, causing it to rearrange within picoseconds to trans-retinal. The change in shape of retinal forces a corresponding change in shape of the protein, rhodopsin, to which it is tightly bound. As a consequence of the protein’s metamorphosis, the behavior of the protein changes in a very specific way. The altered protein can now interact with another protein called transducin. Before associating with rhodopsin, transducin is tightly bound to a small organic molecule called GDP, but when it binds to rhodopsin the GDP dissociates itself from transducin and a molecule called GTP, which is closely related to, but critically different from, GDP, binds to transducin.
The exchange of GTP for GDP in the transducinrhodopsin complex alters its behavior. GTP-transducinrhodopsin binds to a protein called phosphodiesterase, located in the inner membrane of the cell. When bound by rhodopsin and its entourage, the phosphodiesterase acquires the ability to chemically cleave a molecule called cGMP. Initially there are a lot of cGMP molecules in the cell, but the action of the phosphodiesterase lowers the concentration of cGMP. Activating the phosphodiesterase can be likened to pulling the plug in a bathtub, lowering the level of water.
A second membrane protein which binds cGMP, called an ion channel, can be thought of as a special gateway regulating the number of sodium ions in the cell. The ion channel normally allows sodium ions to flow into the cell, while a separate protein actively pumps them out again. The dual action of the ion channel and pump proteins keeps the level of sodium ions in the cell within a narrow range. When the concentration of cGMP is reduced from its normal value through cleavage by the phosphodiesterase, many channels close, resulting in a reduced cellular concentration of positively charged sodium ions. This causes an imbalance of charges across the cell membrane which, finally, causes a current to be transmitted down the optic nerve to the brain: the result, when interpreted by the brain, is vision.
If the biochemistry of vision were limited to the reactions listed above, the cell would quickly deplete its supply of 11-cis-retinal and cGMP while also becoming depleted of sodium ions. Thus a system is required to limit the signal that is generated and restore the cell to its original state; there are several mechanisms which do this. Normally, in the dark, the ion channel, in addition to sodium ions, also allows calcium ions to enter the cell; calcium is pumped back out by a different protein in order to maintain a constant intracellular calcium concentration. However, when cGMP levels fall, shutting down the ion channel and decreasing the sodium ion concentration, calcium ion concentration is also decreased. The phosphodiesterase enzyme, which destroys cGMP, is greatly slowed down at lower calcium concentration. Additionally, a protein called guanylate cyclase begins to resynthesize cGMP when calcium levels start to fall. Meanwhile, while all of this is going on, metarhodopsin II is chemically modified by an enzyme called rhodopsin kinase, which places a phosphate group on its substrate. The modified rhodopsin is then bound by a protein dubbed arrestin, which prevents the rhodopsin from further activating transducin. Thus the cell contains mechanisms to limit the amplified signal started by a single photon.
Trans-retinal eventually falls off of the rhodopsin molecule and must be reconverted to 11-cis-retinal and again bound by opsin to regenerate rhodopsin for another visual cycle. To accomplish this trans-retinal is first chemically modified by an enzyme to transretinol, a form containing two more hydrogen atoms. A second enzyme then isomerizes the molecule to 11-cis-retinol. Finally, a third enzyme removes the previously added hydrogen atoms to form 11-cis-retinal, and the cycle is complete.

|
The three-dimensional structure of rhodopsin. Image courtesy of Wikipedia.
The “explanation” that doesn’t explain
At just one point in their paper do Nilsson and Pelger make any attempt to grapple with the underlying biochemistry of the eye, and that is in their discussion of the evolution of the lens. They write:
The development of a lens with a mathematically ideal distribution of refractive index may at first glance seem miraculous. Yet the elevation of refractive index in the lenses of both vertebrates and cephalopods is caused by proteins that are identical or similar to proteins with other cellular functions (Doolittle 1988; Goldsmith 1990; Winstow and Kim 1991; Land and Fernald 1992). Selection has thus recruited gene products that were already there. Assuming that selection operates on small but random phenotypic variations, no distribution of refractive index is inaccessible to selection.
Now, I don’t wish to contest Nilsson and Pelger’s claim that “Selection has thus recruited gene products that were already there.” Indeed, it turns out that the lenses of both vertebrate and cephalopod eyes are made of a protein called crystallin, and as this paper shows, crystallin is a protein which can be found even in simple sponges. Problem solved, right?
Not so fast. What this simplistic approach overlooks is the fact that crystallin comes in various forms – alpha, beta and gamma crystallin – each of which is strikingly different the others in the way it folds up, as these pictures illustrate. What’s more, the Wikipedia article on crystallin acknowledges that the crystallins used in the lens of the eye are quite different from one another, for different classes of animals:
“The crystallins of different groups of organisms are related to a large number of different proteins, with those from birds and reptiles related to lactate dehydrogenase and argininosuccinate lyase, those of mammals to alcohol dehydrogenase and quinone reductase, and those of cephalopods to glutathione S-transferase and aldehyde dehydrogenase. Whether these crystallins are products of a fortuitous accident of evolution, in that these particular enzymes happened to be transparent and highly-soluble, or whether these diverse enzymatic activities are part of the protective machinery of the lens, is an active research topic.”

|
A box jellyfish has a nerve net, but no brain. Despite this, box jellyfish have no less than 24 eyes, of four different kinds, including true eyes, complete with retinas, corneas and lenses, although their visual resolution is poor. Box jellyfish also display complex, visually guided behaviors such as obstacle avoidance and fast directional swimming. They can also use terrestrial landmarks for navigation. Image courtesy of Wikipedia.
There’s more. The Wikipedia article on crystallin omits to mention cubozoan jellyfish, which also have complex eyes with crystallin lenses, although their resolution is much poorer than the vertebrate eye. A 1993 paper entitled, J1-crystallins of the Cubomedusan Jellyfish Lens Constitute a Novel Family Encoded in at Least Three Intronless Genes by Piatigorsky, Horwitz and Norman (The Journal of Biological Chemistry, Vol. 2643, No. 16, Issue of June 5, 1993, pp. 11894-11901), refers to three different families of proteins in the eyes of these jellyfish, and then goes on to discuss three particular proteins from the first of these families:
“The transparent cellular eye lens of the jellyfish (Tripedalia cystophora) contains three major proteins called J1-, J2-, and J3-crystallins. Here we have isolated cDNAs encoding three novel 37-kDa Jl-crystallin polypeptides (JlA, JlB, and J1C) sharing 84-98% identity in amino acid sequences among themselves.”
The J1-, J2-, and J3-crystallins found in cubozoan jellyfish represent families of proteins. By contrast, the JlA, JlB, and J1C crystallins described in the article above represent three very similar proteins belonging to the same family. (For the benefit of readers who are not familiar with chemical jargon, the 37-kDa figure in the above quote means that the molecule in question is about 37,000 times heavier than a hydrogen atom, or about 3,000 times heavier than a carbon atom. – VJT)
More recent work has shown profound chemical affinities between the J3-crystallin and vertebrate saposins, which are multifunctional proteins that bridge certain enzymes (called hydrolases) to fatty acids called lipids, and which also activate enzyme activity. This striking fact can be readily explained if we suppose that jellyfish and vertebrates share a common ancestor. (See J3-crystallin of the jellyfish lens: Similarity to saposins by Piatigorsky et al., Proceedings of the National Academy of Sciences, Vol. 98, no. 22, October 23, 2001, www.pnas.org/cgi/doi/10.1073/pnas.231310698.)
But while there are indeed surprising similarities between proteins of the same family that may be found in different groups of animals, there are also considerable differences between the various families of proteins which may be found within the same animal. We need to ask: can Darwinian processes account for these differences? For instance, how can we be sure that the J1-, J2-, and J3-crystallins found in cubozoan jellyfish all share a common chemical origin?
Is the inter-conversion between the different kinds of crystallin found in animals’ eyes likely to occur, via Darwinian processes?
At this point, a Darwinist might point to the 84-98% similarity figure between the three versions of J1-crystallin found in cubozoan jellyfish (JlA, JlB, and J1C) and argue, “It’s pretty easy to imagine one form of J1-crystallin converting into another, isn’t it? And given enough time, what is there to prevent inter-conversion between the J1-, J2-, and J3-crystallins found in cubozoans?” However, even a similarity of 84% between two proteins of the same family is not as impressive as it sounds. What it means is that these two proteins, each of which contains several thousand atoms, have hundreds of chemical differences between them. [Update: The differences I’m speaking of here are at the atomic level; in a comment below, Nick Matzke helpfully points out that an 84% similarity would mean about 42 differences in amino acid sequence, or about 21 on each sister lineage – which, as I argue below, still appears to be beyond the reach of unguided evolution.] And this is the point where the numbers really start to matter.
Dr. Douglas Axe’s and Dr. Ann Gauger’s research on the question, “How hard would it be for evolution to produce a different function for a protein?” suggests that it would be virtually impossible (see this video), because six changes is the maximum that evolution can accomplish, during the history of the Earth.
There are good grounds for believing that Darwinian evolution is incapable of transforming a protein that performs one biological function into a different protein which is able to perform a brand new biological function. A recent paper by Dr. Ann K. Gauger and Dr. Douglas D. Axe, entitled, The Evolutionary Accessibility of New Enzyme Functions: A Case Study from the Biotin Pathway (BIO-Complexity 2011(1):1-17. doi:10.5048/BIO-C.2011.1) suggests that such transformations seldom, if ever, occur:
Abstract
Enzymes group naturally into families according to similarity of sequence, structure, and underlying mechanism. Enzymes belonging to the same family are considered to be homologs — the products of evolutionary divergence, whereby the first family member provided a starting point for conversions to new but related functions. In fact, despite their similarities, these families can include remarkable functional diversity. Here we focus not on minor functional variations within families, but rather on innovations — transitions to genuinely new catalytic functions. Prior experimental attempts to reproduce such transitions have typically found that many mutational changes are needed to achieve even weak functional conversion, which raises the question of their evolutionary feasibility. To further investigate this, we examined the members of a large enzyme superfamily, the PLP-dependent transferases, to find a pair with distinct reaction chemistries and high structural similarity. We then set out to convert one of these enzymes, 2-amino-3-ketobutyrate CoA ligase (Kbl2), to perform the metabolic function of the other, 8-amino-7-oxononanoate synthase (BioF2). After identifying and testing 29 amino-acid changes, we found three groups of active-site positions and one single position where Kbl2 side chains are incompatible with BioF2 function. Converting these side chains in Kbl2 makes the residues in the active-site cavity identical to those of BioF2, but nonetheless fails to produce detectable BioF2-like function in vivo. We infer from the mutants examined that successful functional conversion would in this case require seven or more nucleotide substitutions. But evolutionary innovations requiring that many changes would be extraordinarily rare, becoming probable only on timescales much longer than the age of life on earth. Considering that Kbl2 and BioF2 are judged to be close homologs by the usual similarity measures, this result and others like it challenge the conventional practice of inferring from similarity alone that transitions to new functions occurred by Darwinian evolution.
Excerpt from the paper:
The extent to which Darwinian evolution can explain enzymatic innovation seems, on careful inspection, to be very limited. Large-scale innovations that result in new protein folds appear to be well outside its range [5]. This paper argues that at least some small-scale innovations may also be beyond its reach.
Given these biochemical constraints on what Darwinian evolution can accomplish, it is by no means a foregone conclusion that the alpha crystallins present in the crystalline lens of the vertebrate eye could ever have naturally evolved into beta-gamma crystallins, which belong to an entirely different family. Likewise, it is doubtful whether the three families of crystallins (J1, J2, and J3) found in the eyes of cubozoan jellyfish could have developed from a common molecule without intelligent guidance.
These are the “nitty-gritty” questions that Nilsson and Pelger’s 1994 paper fails to address. Even if we grant for that a Darwinian account of the evolution of the eye might work at the anatomical level, it still needs to be shown that it can work at the biochemical level.
I conclude, then, that the 364,000-year estimate proposed by Nilsson and Pelger for the evolution of the eye is not a biologically realistic one: it applies only to a “toy” world where one structure can simply transform itself by imperceptible degrees into another. But without this estimate, the whole foundation for the Darwinian claim that the evolution of the vertebrate eye from a light-sensitive spot is a plausible occurrence collapses. All we are left with is theoretical possibility. And that, as we have seen, isn’t enough to make Darwin’s theory of evolution by natural selection a proper scientific theory.
At the beginning of this post, I posed the question: “Does Nilsson and Pelger’s model lend support to Intelligent Design or Darwinian evolution, or both?” We are now in a position to answer this question fairly definitively. The model was intelligently designed at every step. And while it shows that the eye might well have developed in a gradualistic fashion, it fails to show that natural selection could have accounted for this process. Therefore it provides no support whatsoever to Darwin’s theory of evolution.
Conclusion
As we have seen, there are good reasons for believing that the 364,000-year figure put forward by Nilsson and Pelger for the time required for the eye to have evolved is a fictitious estimate, which has no relevance to biology. However, I don’t wish to sound unduly critical of Nilsson and Pelger’s work: their demonstration that a series of sequential changes, occurring one at a time, could have transformed a light-sensitive spot into a vertebrate eye, was no mean feat, and the acclaim it received was well-deserved. I would also like to add that Dr. Nilsson’s new paper, entitled, “Eye evolution and its functional basis” (forthcoming in Visual Neuroscience, 2013, 30, doi:10.1017/S0952523813000035), marks a major advance on the 1994 paper he co-authored with Dr. Pelger, as it provides a comprehensive account of the evolution of vision in nearly all phyla of animals. Additionally, the new paper attempts to address the evolution of the eye at the biochemical and embryological levels, as well as the morphological level. I intend to discuss Nilsson’s new paper in my next post.